Quartz crystal microbalance with dissipation monitoring of supported lipid bilayers on various substrates
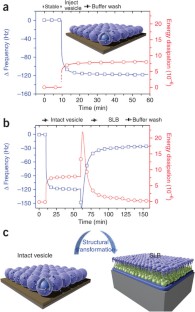
Supported lipid bilayers (SLBs) mimic biological membranes and are a versatile platform for a wide range of biophysical research fields including lipid–protein interactions, protein–protein interactions and membrane-based biosensors. The quartz crystal microbalance with dissipation monitoring (QCM-D) has had a pivotal role in understanding SLB formation on various substrates. As shown by its real-time kinetic monitoring of SLB formation, QCM-D can probe the dynamics of biomacromolecular interactions. We present a protocol for constructing zwitterionic SLBs supported on silicon oxide and titanium oxide, and discuss technical issues that need to be considered when working with charged lipid compositions. Furthermore, we explain a recently developed strategy that uses an amphipathic, α-helical (AH) peptide to form SLBs on gold and titanium oxide substrates. The protocols can be completed in less than 3 h.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
265,23 € per year
only 22,10 € per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
Versatile formation of supported lipid bilayers from bicellar mixtures of phospholipids and capric acid
Article Open access 14 August 2020

Benchtop-fabricated lipid-based electrochemical sensing platform for the detection of membrane disrupting agents
Article Open access 12 March 2020
Carbon-based SERS biosensor: from substrate design to sensing and bioapplication
Article Open access 22 January 2021
References
- Sackmann, E. Supported membranes: scientific and practical applications. Science271, 43–48 (1996). ArticleCASPubMedGoogle Scholar
- Tanaka, M. & Sackmann, E. Supported membranes as biofunctional interfaces and smart biosensor platforms. Physica Status Solidi A203, 3452–3462 (2006). ArticleCASGoogle Scholar
- Hook, F., Kasemo, B., Grunze, M. & Zauscher, S. Quantitative biological surface science: challenges and recent advances. ACS Nano2, 2428–2436 (2008). ArticleCASPubMedGoogle Scholar
- White, R.J. et al. Ionic conductivity of the aqueous layer separating a lipid bilayer membrane and a glass support. Langmuir22, 10777–10783 (2006). ArticleCASPubMedGoogle Scholar
- White, R.J. et al. Single ion-channel recordings using glass nanopore membranes. J. Am. Chem. Soc.129, 11766–11775 (2007). ArticleCASPubMedGoogle Scholar
- Boxer, S.G. Molecular transport and organization in supported lipid membranes. Curr. Opin. Chem. Biol.4, 704–709 (2000). ArticleCASPubMedGoogle Scholar
- Kaiser, H.J. et al. Order of lipid phases in model and plasma membranes. Proc. Natl. Acad. Sci. USA106, 16645–16650 (2009). ArticlePubMedGoogle Scholar
- Hook, F., Rodahl, M., Kasemo, B. & Brzezinski, P. Structural changes in hemoglobin during adsorption to solid surfaces: effects of pH, ionic strength, and ligand binding. Proc. Natl. Acad. Sci. USA95, 12271–12276 (1998). ArticleCASPubMedGoogle Scholar
- Hook, F. et al. Variations in coupled water, viscoelastic properties, and film thickness of a Mefp-1 protein film during adsorption and cross-linking: a quartz crystal microbalance with dissipation monitoring, ellipsometry, and surface plasmon resonance study. Anal. Chem.73, 5796–5804 (2001). ArticleCASPubMedGoogle Scholar
- Cho, N.J., Cheong, K.H., Lee, C., Frank, C.W. & Glenn, J.S. Binding dynamics of hepatitis C virus′ NS5A amphipathic peptide to cell and model membranes. J. Virol.81, 6682–6689 (2007). ArticleCASPubMedPubMed CentralGoogle Scholar
- Cho, N.J., Cho, S.J., Cheong, K.H., Glenn, J.S. & Frank, C.W. Employing an amphipathic viral peptide to create a lipid bilayer on Au and TiO2. J. Am. Chem. Soc.129, 10050–10051 (2007). ArticleCASPubMedGoogle Scholar
- Cooper, M.A. & Singleton, V.T. A survey of the 2001 to 2005 quartz crystal microbalance biosensor literature: applications of acoustic physics to the analysis of biomolecular interactions. J. Mol. Recognit.20, 154–184 (2007). ArticleCASPubMedGoogle Scholar
- Kasemo, B. & Hook, F. Protein and vesicle interaction with surfaces. Abstr. Pap. Am. Chem. Soc.223, U444–U444 (2002). Google Scholar
- Purrucker, O., Fortig, A., Jordan, R., Sackmann, E. & Tanaka, M. Control of frictional coupling of transmembrane cell receptors in model cell membranes with linear polymer spacers. Phys. Rev. Lett.98 (2007).
- Thid, D. et al. Supported phospholipid bilayers as a platform for neural progenitor cell culture. J. Biomed. Mater. Res.84, 940–953 (2008). ArticleCASGoogle Scholar
- Tu, R.S. & Tirrell, M. Bottom-up design of biomimetic assemblies. Adv. Drug Deliv. Rev.56, 1537–1563 (2004). ArticleCASPubMedGoogle Scholar
- Tamm, L.K. & McConnell, H.M. Supported phospholipid bilayers. Biophys. J.47, 105–113 (1985). ArticleCASPubMedPubMed CentralGoogle Scholar
- Axelrod, D., Koppel, D.E., Schlessinger, J., Elson, E. & Webb, W.W. Mobility measurement by analysis of fluorescence photobleaching recovery kinetics. Biophys. J.16, 1055–1069 (1976). ArticleCASPubMedPubMed CentralGoogle Scholar
- Richter, R., Mukhopadhyay, A. & Brisson, A. Pathways of lipid vesicle deposition on solid surfaces: a combined QCM-D and AFM study. Biophys. J.85, 3035–3047 (2003). ArticleCASPubMedPubMed CentralGoogle Scholar
- Morigaki, K. & Tawa, K. Vesicle fusion studied by surface plasmon resonance and surface plasmon fluorescence spectroscopy. Biophys. J.91, 1380–1387 (2006). ArticleCASPubMedPubMed CentralGoogle Scholar
- Reimhult, E., Larsson, C., Kasemo, B. & Hook, F. Simultaneous surface plasmon resonance and quartz crystal microbalance with dissipation monitoring measurements of biomolecular adsorption events involving structural transformations and variations in coupled water. Anal. Chem.76, 7211–7220 (2004). ArticleCASPubMedGoogle Scholar
- Oxhamre, C., Richter-Dahlfors, A., Zhdanov, V.P. & Kasemo, B. A minimal generic model of bacteria-induced intracellular Ca2+ oscillations in epithelial cells. Biophys. J.88, 2976–2981 (2005). ArticleCASPubMedGoogle Scholar
- Stroumpoulis, D., Parra, A. & Tirrell, M. A kinetic study of vesicle fusion on silicon dioxide surfaces by ellipsometry. AIChE J.52, 2931–2937 (2006). ArticleCASGoogle Scholar
- Cho, N.J. et al. Alpha-helical peptide-induced vesicle rupture revealing new insight into the vesicle fusion process as monitored in situ by quartz crystal microbalance-dissipation and reflectometry. Anal. Chem.81, 4752–4761 (2009). ArticleCASPubMedGoogle Scholar
- Wang, G. et al. A combined reflectometry and quartz crystal microbalance with dissipation setup for surface interaction studies. Rev. Sci. Instrum.79, 075107 (2008). ArticleCASPubMedGoogle Scholar
- Anderson, T.H. et al. Formation of supported bilayers on silica substrates. Langmuir25, 6997–7005 (2009). ArticleCASPubMedGoogle Scholar
- Kanazawa, K. & Gordon, J. The oscillation frequency of a quartz resonator in contact with a liquid. Anal. Chim. Acta175, 99–106 (1985). ArticleCASGoogle Scholar
- Kanazawa, K.K. & Reed, C.E. A new description for the viscoelastically loaded quartz resonator. Abstr. Pap. Am. Chem. Soc.198, 89 Anyl (1989). Google Scholar
- Rodahl, M. et al. Simultaneous frequency and dissipation factor QCM measurements of biomolecular adsorption and cell adhesion. Faraday Discuss.107, 229–246 (1997). ArticleCASGoogle Scholar
- Rodahl, M., Hook, F. & Kasemo, B. QCM operation in liquids: an explanation of measured variations in frequency and Q factor with liquid conductivity. Anal. Chem.68, 2219–2227 (1996). ArticleCASPubMedGoogle Scholar
- Rodahl, M., Hook, F., Krozer, A., Brzezinski, P. & Kasemo, B. Quartz-crystal microbalance setup for frequency and q-factor measurements in gaseous and liquid environments. Rev. Sci. Instrum.66, 3924–3930 (1995). ArticleCASGoogle Scholar
- Rodahl, M. & Kasemo, B. Frequency and dissipation-factor responses to localized liquid deposits on a QCM electrode. Sens. Actuators B Chem.37, 111–116 (1996). ArticleCASGoogle Scholar
- Rodahl, M. & Kasemo, B. On the measurement of thin liquid overlayers with the quartz-crystal microbalance. Sens. Actuators A Phys.54, 448–456 (1996). ArticleCASGoogle Scholar
- Rodahl, M. & Kasemo, B. A simple setup to simultaneously measure the resonant frequency and the absolute dissipation factor of a quartz crystal microbalance. Rev. Sci. Instrum.67, 3238–3241 (1996). ArticleCASGoogle Scholar
- Cho, N.J., Kanazawa, K.K., Glenn, J.S. & Frank, C.W. Employing two different quartz crystal microbalance models to study changes in viscoelastic behavior upon transformation of lipid vesicles to a bilayer on a gold surface. Anal. Chem.79, 7027–7035 (2007). ArticleCASPubMedGoogle Scholar
- Hook, F. & Kasemo, B. The QCM-D technique for probing biomacromolecular recognition reactions. Springer Ser. Chem. Sens. Biosens.5, 425–447 (2007). ArticleCASGoogle Scholar
- Keller, C.A. & Kasemo, B. Surface specific kinetics of lipid vesicle adsorption measured with a quartz crystal microbalance. Biophys. J.75, 1397–1402 (1998). ArticleCASPubMedPubMed CentralGoogle Scholar
- Keller, C.A., Glasmastar, K., Zhdanov, V.P. & Kasemo, B. Formation of supported membranes from vesicles. Phys. Rev. Lett.84, 5443–5446 (2000). ArticleCASPubMedGoogle Scholar
- Lee, S.E. et al. Biologically functional cationic phospholipid-gold nanoplasmonic carriers of RNA. J. Am. Chem. Soc.131, 14066–14074 (2009). ArticleCASPubMedGoogle Scholar
- Larsson, E.M., Edvardsson, M.E., Langhammer, C., Zoric, I. & Kasemo, B. A combined nanoplasmonic and electrodeless quartz crystal microbalance setup. Rev. Sci. Instrum.80, 125105 (2009). ArticleCASPubMedGoogle Scholar
- Jonsson, M.P., Jonsson, P. & Hook, F. Simultaneous nanoplasmonic and quartz crystal microbalance sensing: analysis of biomolecular conformational changes and quantification of the bound molecular mass. Anal. Chem.80, 7988–7995 (2008). ArticleCASPubMedGoogle Scholar
- Jonsson, M.P., Jonsson, P., Dahlin, A.B. & Hook, F. Supported lipid bilayer formation and lipid-membrane-mediated biorecognition reactions studied with a new nanoplasmonic sensor template. Nano Lett.7, 3462–3468 (2007). ArticleCASPubMedGoogle Scholar
- Misra, N. et al. Bioelectronic silicon nanowire devices using functional membrane proteins. Proc. Natl Acad. Sci. USA106, 13780–13784 (2009). ArticlePubMedGoogle Scholar
- Kasemo, B. & Lausmaa, J. Material-tissue interfaces: the role of surface properties and processes. Environ. Health Perspect.102 (Suppl 5): 41–45 (1994). ArticlePubMedPubMed CentralGoogle Scholar
- Seifert, U., Berndl, K. & Lipowsky, R. Shape transformations of vesicles—phase-diagram for spontaneous-curvature and bilayer-coupling models. Phys. Rev. A44, 1182–1202 (1991). ArticleCASPubMedGoogle Scholar
- Shillcock, J.C. & Lipowsky, R. Tension-induced fusion of bilayer membranes and vesicles. Nat. Mater.4, 225–228 (2005). ArticleCASPubMedGoogle Scholar
- Polozov, I.V., Anantharamaiah, G.M., Segrest, J.P. & Epand, R.M. Osmotically induced membrane tension modulates membrane permeabilization by class L amphipathic helical peptides: nucleation model of defect formation. Biophys. J.81, 949–959 (2001). ArticleCASPubMedPubMed CentralGoogle Scholar
- Lasic, D.D. The mechanism of vesicle formation. Biochem. J.256, 1–11 (1988). ArticleCASPubMedPubMed CentralGoogle Scholar
- Lasic, D.D. & Martin, F.J. On the mechanism of vesicle formation. J. Memb. Sci.50, 215–222 (1990). ArticleCASGoogle Scholar
- Watwe, R.M. & Bellare, J.R. Manufacture of liposomes—a review. Curr. Sci.68, 715–724 (1995). CASGoogle Scholar
- Winterhalter, M. & Lasic, D.D. Liposome stability and formation—experimental parameters and theories on the size distribution. Chem. Phys. Lipids64, 35–43 (1993). ArticleCASPubMedGoogle Scholar
- Armengol, X. & Estelrich, J. Physical stability of different liposome compositions obtained by extrusion method. J. Microencapsul.12, 525–535 (1995). ArticleCASPubMedGoogle Scholar
- Shingles, R. & McCarty, R.E. Production of membrane vesicles by extrusion: size distribution, enzyme activity, and orientation of plasma membrane and chloroplast inner-envelope membrane vesicles. Anal. Biochem.229, 92–98 (1995). ArticleCASPubMedGoogle Scholar
- Seantier, B. & Kasemo, B. Influence of mono- and divalent ions on the formation of supported phospholipid bilayers via vesicle adsorption. Langmuir25, 5767–5772 (2009). ArticleCASPubMedGoogle Scholar
- Kasemo, B. Biocompatibility of titanium implants: surface science aspects. J. Prosthet. Dent.49, 832–837 (1983). ArticleCASPubMedGoogle Scholar
- Kasemo, B. & Lausmaa, J. Aspects of surface physics on titanium implants. Swed. Dent. J. Suppl.28, 19–36 (1985). CASPubMedGoogle Scholar
- Greve, F. et al. Molecular design and characterization of the neuron-microelectrode array interface. Biomaterials28, 5246–5258 (2007). ArticleCASPubMedGoogle Scholar
- Reimhult, E., Hook, F. & Kasemo, B. Vesicle adsorption on SiO2 and TiO2: Dependence on vesicle size. J. Chem. Phys.117, 7401–7404 (2002). ArticleCASGoogle Scholar
- Modin, C. et al. QCM-D studies of attachment and differential spreading of pre-osteoblastic cells on Ta and Cr surfaces. Biomaterials27, 1346–1354 (2006). ArticleCASPubMedGoogle Scholar
- Ekeroth, J., Konradsson, P. & Höök, F. Bivalent-ion-mediated vesicle adsorption and controlled supported phospholipid bilayer formation on molecular phosphate and sulfate layers on gold. Langmuir18, 7923–7929 (2002). ArticleCASGoogle Scholar
- Rossetti, F.F., Textor, M. & Reviakine, I. Asymmetric distribution of phosphatidyl serine in supported phospholipid bilayers on titanium dioxide. Langmuir22, 3467–3473 (2006). ArticleCASPubMedGoogle Scholar
- Rossetti, F.F., Bally, M., Michel, R., Textor, M. & Reviakine, I. Interactions between titanium dioxide and phosphatidyl serine-containing liposomes: formation and patterning of supported phospholipid bilayers on the surface of a medically relevant material. Langmuir21, 6443–6450 (2005). ArticleCASPubMedGoogle Scholar
- Kunze, A., Sjovall, P., Kasemo, B. & Svedhem, S. In situ preparation and modification of supported lipid layers by lipid transfer from vesicles studied by QCM-D and TOF-SIMS. J. Am. Chem. Soc.131, 2450–2451 (2009). ArticleCASPubMedGoogle Scholar
- Jackman, J.A., Cho, N.J., Duran, R.S. & Frank, C.W. Interfacial binding dynamics of bee venom phospholipase A(2) investigated by dynamic light scattering and quartz crystal microbalance. Langmuir26, 4103–4112 (2010). ArticleCASPubMedGoogle Scholar
- Cho, N.J. et al. Quartz resonator signatures under Newtonian liquid loading for initial instrument check. J. Colloid Interface Sci.315, 248–254 (2007). ArticleCASPubMedGoogle Scholar
- Reimhult, E., Hook, F. & Kasemo, B. Temperature dependence of formation of a supported phospholipid bilayer from vesicles on SiO2. Phy. Rev. E66 (2002).
- Reimhult, E., Hook, F. & Kasemo, B. Intact vesicle adsorption and supported biomembrane formation from vesicles in solution: Influence of surface chemistry, vesicle size, temperature, and osmotic pressure. Langmuir19, 1681–1691 (2003). ArticleCASGoogle Scholar
- Zhdanov, V.P., Dimitrievski, K. & Kasemo, B. Adsorption and spontaneous rupture of vesicles composed of two types of lipids. Langmuir22, 3477–3480 (2006). ArticleCASPubMedGoogle Scholar
- Dimitrievski, K., Reimhult, E., Kasemo, B. & Zhdanov, V.P. Simulations of temperature dependence of the formation of a supported lipid bilayer via vesicle adsorption. Colloids Surf. B Biointerfaces39, 77–86 (2004). ArticleCASPubMedGoogle Scholar
- Dimitrievski, K. & Kasemo, B. Influence of lipid vesicle composition and surface charge density on vesicle adsorption events: a kinetic phase diagram. Langmuir25, 8865–8869 (2009). ArticleCASPubMedGoogle Scholar
- Sauerbrey, G. Verwendung von Schwingquarzen zur Wagung dunner Schichten und zur Mikrowagung. Z. Phys.155, 206–222 (1959). ArticleCASGoogle Scholar
- Voinova, M.V., Rodahl, M., Jonson, M. & Kasemo, B. Viscoelastic acoustic response of layered polymer films at fluid-solid interfaces: continuum mechanics approach. Phys. Scr.59, 391–396 (1999). ArticleCASGoogle Scholar
- Johannsmann, D., Reviakine, I. & Richter, R.P. Dissipation in films of adsorbed nanospheres studied by quartz crystal microbalance (QCM). Anal. Chem.81, 8167–8176 (2009). ArticleCASPubMedGoogle Scholar
- Richter, R.P. & Brisson, A.R. Following the formation of supported lipid bilayers on mica: a study combining AFM, QCM-D, and ellipsometry. Biophys. J.88, 3422–3433 (2005). ArticleCASPubMedPubMed CentralGoogle Scholar
- Kasemo, B. & Lausmaa, J. Biomaterial and implant surfaces: on the role of cleanliness, contamination, and preparation procedures. J. Biomed. Mater. Res.22 (A2 Suppl): 145–158 (1988). ArticleCASPubMedGoogle Scholar
- Hook, F. et al. A comparative study of protein adsorption on titanium oxide surfaces using in situ ellipsometry, optical waveguide lightmode spectroscopy, and quartz crystal microbalance/dissipation. Colloids Surf. B Biointerfaces24, 155–170 (2002). ArticleCASGoogle Scholar
- Mashaghi, A., Swann, M., Popplewell, J., Textor, M. & Reimhult, E. Optical anisotropy of supported lipid structures probed by waveguide spectroscopy and its application to study of supported lipid bilayer formation kinetics. Anal. Chem.80, 3666–3676 (2008). ArticleCASPubMedGoogle Scholar
- Richter, R.P., Maury, N. & Brisson, A.R. On the effect of the solid support on the interleaflet distribution of lipids in supported lipid bilayers. Langmuir21, 299–304 (2005). ArticleCASPubMedGoogle Scholar
- Stelzle, M. & Sackmann, E. Sensitive detection of protein adsorption to supported lipid bilayers by frequency-dependent capacitance measurements and microelectrophoresis. Biochim. Biophys. Acta.981, 135–142 (1989). ArticleCASPubMedGoogle Scholar
- Cho, N.J., Cho, S.J., Hardesty, J.O., Glenn, J.S. & Frank, C.W. Creation of lipid partitions by deposition of amphipathic viral peptides. Langmuir23, 10855–10863 (2007). ArticleCASPubMedGoogle Scholar
- Meyvis, T.K., De Smedt, S.C., Van Oostveldt, P. & Demeester, J. Fluorescence recovery after photobleaching: a versatile tool for mobility and interaction measurements in pharmaceutical research. Pharm. Res.16, 1153–1162 (1999). ArticleCASPubMedGoogle Scholar
- Bailey, L.E. et al. Multistep adsorption of perfluoropolyether hard-disk lubricants onto amorphous carbon substrates from solution. Langmuir17, 8145–8155 (2001). ArticleCASGoogle Scholar
- Bobardt, M.D. et al. Hepatitis C virus NS5A anchor peptide disrupts human immunodeficiency virus. Proc. Natl Acad. Sci. USA105, 5525–5530 (2008). ArticlePubMedGoogle Scholar
- Cho, N.J. et al. The mechanism of an amphipathic α-helical peptide's antiviral activity involves size-dependent virus particle lysis. ACS Chem. Biol.4, 1061–1067 (2009). ArticleCASPubMedGoogle Scholar
- Han, X. et al. Supported bilayer lipid membrane arrays on photopatterned self-assembled monolayers. Chemistry13, 7957–7964 (2007). ArticleCASPubMedGoogle Scholar
- Purrucker, O. et al. Polymer-tethered membranes as quantitative models for the study of integrin-mediated cell adhesion. Soft Matter3, 333–336 (2007). ArticleCASGoogle Scholar
- Sklan, E.H. et al. A Rab-GAP TBC domain protein binds hepatitis C virus NS5A and mediates viral replication. J. Virol.81, 11096–11105 (2007). ArticleCASPubMedPubMed CentralGoogle Scholar
- Yu, X. et al. Cryo-electron microscopy and three-dimensional reconstructions of hepatitis C virus particles. Virology367, 126–134 (2007). ArticleCASPubMedGoogle Scholar
Acknowledgements
N.-J.C. is a recipient of an American Liver Foundation Postdoctoral Fellowship Award and a Global Roche Postdoctoral Fellowship. We wish to thank all the members of the Frank, Kasemo and Hook laboratories, who laid the foundation for future studies in the biomimetic sensor field.
Author information
Authors and Affiliations
- Division of Gastroenterology and Hepatology, Department of Medicine, Stanford University, Stanford, California, USA Nam-Joon Cho
- Department of Chemical Engineering, Stanford University, Stanford, California, USA Nam-Joon Cho & Curtis W Frank
- Department of Applied Physics, Chalmers University of Technology, Gothenburg, Sweden Bengt Kasemo & Fredrik Höök
- Nam-Joon Cho









